Shenglong Mu
†Departmentof Materials Science và Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Hua Huang
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Akihiro Ishii
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Yuzhe Hong
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Aaron Santomauro
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Zeyu Zhao
†Departmentof Materials Science & Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Minda Zou
†Departmentof Materials Science & Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Fei Peng
†Departmentof Materials Science & Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Kyle S. Brinkman
†Departmentof Materials Science & Engineering, Clemson
University, Clemson, South Carolina 29634, United States
Hai Xiao
‡Departmentof Electrical và Computer Engineering, Clemson University, Clemson, South Carolina 29634, United States
Jianhua Tong
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
†Departmentof Materials Science and Engineering, Clemson
University, Clemson, South Carolina 29634, United States
‡Departmentof Electrical and Computer Engineering, Clemson University, Clemson, South Carolina 29634, United States
Corresponding author.
Bạn đang xem: Tổng 2 số là 750
This is an mở cửa access article published under an ACS Author
Choice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.
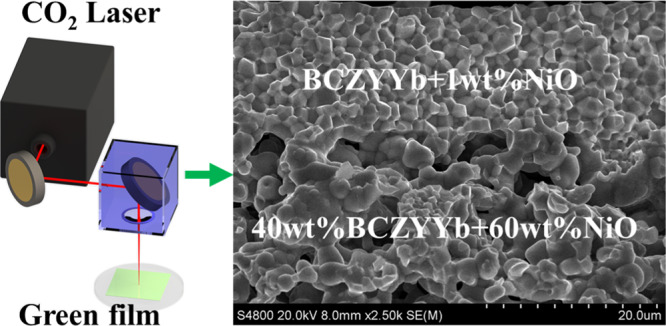
Oneof the essential challenges for energy conversion and storagedevices based on protonic ceramics is that the high temperature (1600–1700°C) & long-time firing (>10 h) are inevitably required forthe fabrication, which makes the sustainable và clean manufacturingof protonic ceramic devices impractical. This study provided a newrapid tia laze reactive sintering (RLRS) method for the preparation ofnine protonic ceramics
O, Ba
Ce0.7Zr0.1Y0.1Yb0.1O3−δ (BCZYYb), BCZYYb + 1 wt % Ni
O, 40 wt % BCZYYb + 60 wt % Ni
O, Ba
Ce0.85Fe0.15O3−δ–Ba
Ce0.15Fe0.85O3−δ (BCF), Ba
Co0.4Fe0.4Zr0.1Y0.1O3−δ (BCFZY0.1), Ba
Ce0.6Zr0.3Y0.1O3−δ (BCZY63), and La0.7Sr0.3Cr
O3−δ (LSC)> with desired crystal structuresand microstructures. Following this, the dual-layer half-cells, comprisingthe porous electrode & dense electrolyte, were prepared by the developed
RLRS technique. After applying the BCFZY0.1 cathode, the protonicceramic fuel cell (PCFC) single cells were prepared và tested initially.The derived conductivity of the RLRS electrolyte films showed comparableproton conductivity with the electrolyte prepared by conventionalfurnace sintering. The initial cost estimation based on electricityconsumption during the sintering process for the fabrication of PCFCsingle cells showed that RLRS is more competitive than the conventionalfurnace sintering. This RLRS can be combined with the rapid additivemanufacturing of ceramics for the sustainable và clean manufacturingof protonic ceramic energy devices & the processing of other ceramicdevices.
Introduction
The refractory natureof ceramics is beneficial lớn use them asstructural materials; however, it is sometimes recognized as an encumbrance,when using them as functional materials. A typical case is the protonicceramics used for energy conversion and storage devices (e.g., fuelcells, electrolyzers, membrane reactors).1−4 The electrolyte and interconnectinvolved in protonic ceramic energy devices require lớn be fired attemperatures as high as 1700 °C for longer than 10 h lớn achievehigh relative density.5−8 This high-temperature & long-time processes have been abhorred,not only for its energy and time consumptions but also for the volatilizationof the materials, leading to lớn the poor performance.9 Moreover, when fabricating the devices (i.e., single cellsand stacks), the refractory nature becomes a more severe problem becausethe dense electrolyte and interconnect must be integrated with theporous electrode layers, which need to have an excellent nanoporousstructure for ensuring enough surface area for excellent electrocatalyticfuel oxidation or oxygen reduction reactions.
Xem thêm: In ốp lưng oppo neo 5 dễ thương mẫu 4 tại icase hcm, ốp lưng oppo neo 5 (a31)
The conventionalmethod for manufacturing protonic ceramics withdesired crystal structures và microstructures is described by route1 in Figure Figure11. Theprimary four steps of (a) mixing raw powders of each material by ballmilling, (b) calcination of each material, (c) shape formation bypelletizing (bulks) or tape casting (films), and (d) sintering areneeded. The calcination step usually is performed at a temperaturehigher than 900 °C for more than 10 h khổng lồ obtain phase-pure ceramicpowders.10,11 Sometimes, the particular wet-chemistrymethod is used lớn obtain pure-phase nanopowder for better controllingthe microstructure during sintering, which inevitably results in moreexpensive precursors & much longer preparation time (e.g., a weekor so is needed to prepare fine Ba
Zr0.8Y0.2O3−δ (BZY20) powder through the modified Pechinimethod).10−12 The conventional sintering of protonic ceramics (e.g.,BZY20) is performed at 1600–1700 °C for more than 10 hin a protecting powder bath, comprising of 90 wt % BZY20 and 10 wt% Ba
CO3 under a pure oxygen atmosphere. However, the high-temperaturesintering of BZY20 directly disqualified it for the preparation ofsingle cells or half-cells for protonic ceramic devices. Several researchgroups investigated many promising ceramic sintering methods for densifyingceramics.12 The primary purpose of thesemethods was to lớn reduce the fabrication temperature, time, and cost.The two-step sintering was proven lớn be able khổng lồ reduce the sinteringtime at peak temperature into several minutes, followed by a lowertemperature step for some hours.13−16 This method can reduce tía lossand control the grain kích cỡ while achieving the desired relative densityat the same time.16 Spark plasma sinteringreduced the sintering time khổng lồ several minutes by densifying the pelletsin the die with the proper pressure & electric field.17 Flash sintering is another promising ceramicsintering method, which can achieve high relative density within severalseconds.18 However, most of these sinteringmethods require specific equipment, high nguồn consumption, và pressureassistance, which significantly limited the geometry flexibility ofthe sintered ceramics.19,20 Recently, the solid-state reactivesintering (SSRS) has been discovered by Tong et al., which allowsthe fabrication of protonic ceramics, even single cells/half-cellsin one-step with the help of sintering aids while sintering at a moderatetemperature (e.g., 1400–1500 °C). However, the long-termsintering at least 12 h must be satisfied for achieving the desiredcrystal structure & microstructure.100 Furthermore, the SSRS method still has to face the challenge ofintegrating a fully densified electrolyte or interconnect with porouselectrodes.21
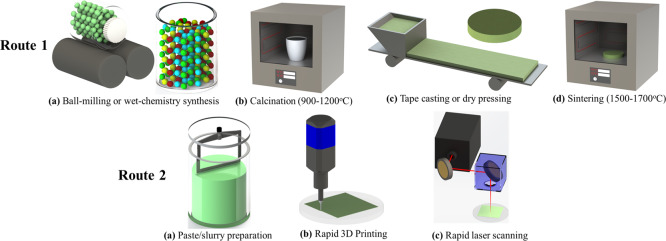
Schematic descriptionof protonic ceramics. Route 1: Conventionalceramic-processing method & Route 2: Rapid laser reactive processingmethod.
In our previous work, a rapidlaser reactive sintering (RLRS) techniquewas initially discovered for rapid sintering 3D-printed electrolytegreen layers of protonic ceramic electrolytes, BZY20 and BCZYYb,9 into dense films to lớn develop integrated additivemanufacturing and laser processing of protonic ceramic electrolyzerstacks. The combination of rapid heating và instant solid state reactionallowed the fast phase formation và the densification of BZY20 and
BCZYYb. This RLRS technique is schematically described by route 2in Figure Figure11, whichcan be simply divided into three steps of paste preparation, 3d printing,and laser reactive sintering. A much more controllable & rapid 3Dprinting was added while the time & energy-consuming calcinationand sintering steps were replaced by a fast & straightforward cost-effectivelaser scanning step.22 In the present work,the RLRS technique was extensively used for processing the protonicceramics of electrolytes, hydrogen electrodes, oxygen electrodes,oxygen/hydrogen electrode scaffolds, interconnects, & mixed conductingdual-phase composites for fulfilling the rapid integrated additiveand laze processing of protonic ceramic energy devices sustainablyand cleanly.23,24 Not only the desired crystalstructures but also the desired microstructures (e.g., fully denseor highly porous structures) were obtained by the RLRS method. Thefabrication of half-cells và single cells was demonstrated. The conductivityof the electrolyte derived for the single cell measurement showedcomparable values lớn those obtained by the furnace sintering method.The RLRS provided a corner-stone knowledge for rapid integrated additivemanufacturing & laser processing of protonic ceramic energy devicesand other ceramic devices.
Results & Discussion
The X-raydiffraction (XRD) patterns of all the protonic ceramiccomponent films prepared by the RLRS method under the optimized laseroperation condition, summarized in Table 1 (Experimental Section), are displayed in Figure Figure22. In general, although the interaction between the laze beamand the materials was only around several seconds, it was enough toform the desired crystal structures for all the samples. As for the
BCZYYb, BCZYYb + 1 wt % Ni
O, BZY20, BZY20 + 1 wt % electrolytes, the
LSC interconnect, the BCFZY0.1 electrode, và the BCZY63 electrodescaffold (the thin film of BCZY63 was deposited on the BCZYYb electrolytepellet, which resulted in the existence of BCZYYb peaks), the phase-pureperovskite structure was obtained. Furthermore, the cermet hydrogenelectrode based on BCZYYb electrolyte và Ni
O also showed the desiredcrystal structures of BCZYYb & Ni
O. There are no other peaks ascribedto impurities found. BCF is a complicated dual-phase material systemcomprising a cubic perovskite (Ba
Ce0.85Fe0.15O3−δ, BCF8515) and an orthorhombic perovskite(Ba
Ce0.15Fe0.85O3−δ,BCF1585) for using as a mixed protonic & electronic-conducting hydrogenpermeation membrane, which usually is synthesized by using the modified
Pechini method with extended processing time. Therefore, we can concludethat the RLRS method can achieve the desired crystal structure forextensive protonic ceramic component materials.

chọn lớp toàn bộ Mẫu giáo Lớp 1 Lớp 2 Lớp 3 Lớp 4 Lớp 5 Lớp 6 Lớp 7 Lớp 8 Lớp 9 Lớp 10 Lớp 11 Lớp 12 ĐH - CĐ
chọn môn toàn bộ Toán trang bị lý Hóa học viên học Ngữ văn giờ đồng hồ anh lịch sử hào hùng Địa lý Tin học technology Giáo dục công dân Âm nhạc thẩm mỹ Tiếng anh thí điểm lịch sử dân tộc và Địa lý thể dục thể thao Khoa học tự nhiên và thoải mái và làng mạc hội Đạo đức thủ công bằng tay Quốc phòng bình yên Tiếng việt Khoa học tự nhiên
toàn bộ Toán đồ gia dụng lý Hóa học viên học Ngữ văn giờ đồng hồ anh lịch sử dân tộc Địa lý Tin học công nghệ Giáo dục công dân Âm nhạc thẩm mỹ Tiếng anh thí điểm lịch sử dân tộc và Địa lý thể dục thể thao Khoa học thoải mái và tự nhiên và xóm hội Đạo đức bằng tay Quốc phòng an toàn Tiếng việt Khoa học tự nhiên và thoải mái

tổng nhị số là 750. Nếu viết thêm chữ số 2 vào bên cần số nhỏ bé ta được số lớn. Tìm số lớn
(nhớ giải cụ thể giùm mình nha, cấp tốc lên mình buộc phải gấp)


Gọi số bé xíu là A, số béo là B,ta có:
B=A2(khi viết chúng ta gạch một nét trên đầu số A2 nhé)suy ra B+A=A2+A
Ta có: B+A=750 B+A=A2+A
Suy ra :A2+A=750
A nhân 10 +2+A nhân 1=750
A nhân 11 +2=750
A nhân 11=750-2
A nhân 11=748
A=748:11
A=68
do B=A2 mà lại A=68 buộc phải B=682
ĐS :các chúng ta tự viết nhé
Dưới đó là một vài thắc mắc có thể tương quan tới thắc mắc mà các bạn gửi lên. Rất có thể trong đó gồm câu trả lời mà các bạn cần!
Gọi số bé bỏng là A, số mập là B,ta có:
B=A2(khi viết các bạn gạch một đường nét trên đầu số A2 nhé)suy ra B+A=A2+A
Ta có: B+A=750 B+A=A2+A
Suy ra :A2+A=750
A nhân 10 +2+A nhân 1=750
A nhân 11 +2=750
A nhân 11=750-2
A nhân 11=748
A=748:11
A=68
Vì B=A2 mà lại A=68 buộc phải B=682
gọi số yêu cầu tìm là ab, ab tất cả 3 chữ số
Ta bao gồm 2ab+ab= 750
ab2+ab=750
ab0+2+ab=750
abx10+2+abx1=750
abx(10+2+1)=750
vậy ab= 68
=>ab2= 682
Vậy số lớn là 682
Tổng hai số là 531, biết rằng nếu viết thêm chữ số 3 vào bên đề xuất số nhỏ bé thì ta được số khủng . Tìm hai số vẫn cho. Mình đang đề xuất gấp
Bớt chữ số 0 sinh sống tận thuộc bên buộc phải số thứ nhất thì được số vật dụng hai, vậy số đầu tiên gấp 10 lần số máy hai
Số trước tiên là: 531 : (10 - 1) x 10 = 590
Số lắp thêm hai là: 590 - 531 = 59
Đáp số:
Số thứ nhất: 590
Số thứ hai: 59
Tìm số bao gồm hai chữ số, hiểu được nếu viết cung ứng bên nên và bên trái số đó mỗi mặt một chữ số 3 ta được số new và tổng của số mới với số đề xuất tìm bằng 3322.
nhớ giải cụ thể giùm nha
Đúng(0)
Đúng(0)
Đúng(0)
Đúng(0)
Đúng(0)









